How an enzyme uses sunlight for DNA repair
DNA damage drives cancer, ageing, and cell death. Therefore, DNA repair is crucial for all organisms, and a deeper understanding of this basic function helps us better comprehend how life around us survives and thrives. An international team of researchers led by CFEL @ DESY and with participation of Universität Hamburg and European XFEL reports in the current issue of Science their use of time-resolved crystallography to understand how the enzyme photolyase efficiently channels the energy of sunlight into DNA repair chemistry.
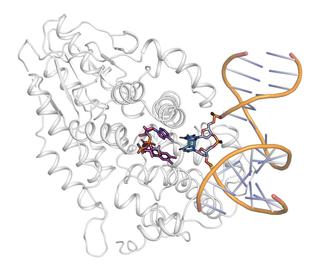
Illustration of the structure of the photolyase with bound DNA (yellow and blue) and the photochemically active area (purple). Image: DESY
All life under the sun must cope with harmful UV rays. UV damage can take many forms, but DNA, the molecule that carries the genetic information of all living organisms, is especially vulnerable. For instance, UV can drive chemical cross-linking reactions of DNA, potentially introducing errors into the genetic code. This cross-linking can lead to cell death or – in the worst cases – mutagenesis and cancer. Such damage is not uncommon; under bright sunlight, a human skin cell can undergo 50-100 cross linking reactions per second.
“To survive, life has evolved powerful DNA repair mechanisms. One especially elegant solution is provided by the enzyme photolyase,” explains DESY scientist Thomas J. Lane, who is a researcher in the Center for Free-Electron Laser Science CFEL and in the Cluster of Excellence “CUI: Advanced Imaging of Matter” at Universität Hamburg. The enzyme uses sunlight to repair damage caused by sunlight. Photolyase is able to recognize the location where UV irradiation has cross-linked DNA and grabs onto those bits of damaged DNA. Then, it can capture a blue photon from the sun, and use it to perform repair chemistry, turning the DNA back into its original, healthy form.
To better understand how photolyase works, the scientists were particularly interested first in the form of the enzyme immediately after absorbing a photon, but before repairing the DNA. Second, they wanted to find out the exact sequence of bond-breaking chemical reactions necessary to turn damaged DNA into healthy DNA. As a third step, the team sought to better understand how photolyase can specifically recognize which DNA is damaged.
This research was possible thanks to the recent development of X-ray free-electron laser sources. The picture shows the set-up at the SwissFEL X-ray free-electron laser, where the ultrashort X-ray pulses necessary to analyze the DNA repair mechanism were generated. Photo: DESY, A. Pateras
Conducting time-resolved crystallography at the SwissFEL X-ray free-electron laser in Villigen, Switzerland, the scientists were able to capture the excited state of the photolyase chromophore, letting them understand how the enzyme efficiently channels the energy of sunlight into DNA repair chemistry. “This research was only made possible by the recent development of X-ray free-electron laser sources. Their intense femtosecond-duration pulses let us record flash X-ray photographs that freeze all atomic motion so that we can follow the reaction step by step at the speed of molecules,” says first author Nina-Eleni Christou from DESY.
Further, for decades, it was debated if the two carbon-carbon bonds involved in the repair process broke simultaneously, or in a stepwise fashion. Seeing that one bond breaks first, followed by the second, the team could finally answer this question. Finally, the enzyme has a binding pocket that is perfectly matched to the shape of damaged DNA. The researchers found that the repaired DNA does not fit in this pocket, as it’s too large and has the wrong shape. This explains why photolyase has a higher affinity for the damaged DNA than repaired, healthy DNA.
The scientists determined ten time-resolved structures of photolyase in the act of DNA repair. “Together, our structures illuminate the function of a powerful DNA repair system that elegantly uses sunlight to repair damage caused by sunlight,” Thomas J. Lane concludes. Unfortunately, humans lack this enzyme and must rely on other DNA repair mechanisms, but photolyase plays an important DNA repair role for nearly all other species.
The work was funded by the following: Helmholtz Association, Universität Hamburg, Swiss National Science Foundation, Göran Gustafsson Foundation, Carl Trygger Foundation, Imperial College London, Cluster of Excellence “CUI: Advanced Imaging of Matter” at Universität Hamburg, National Science Foundation (USA), Department of Energy (USA).