Reaction rate of many molecules depends on their shape
Using a sorting machine for molecules, a German–Swiss research team can now for the first time directly measure the various reaction rates of different forms of the same compound.
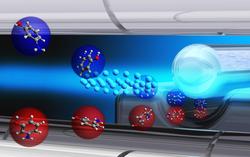
Artist's concept of the sorting mechanism. Conformers with a larger dipole moment (marked blue) are deflected stronger than those with a smaller dipole moment (marked red). This way, one type of conformers can be picked and lead seperately to react with the ultra cool calcium ions. Image: Yuan-Pin Chang/CFEL-DESY
Most molecules occur in several shapes, which may behave very differently. Using a sorting machine for molecules, a German–Swiss research team can now for the first time directly measure the various reaction rates of different forms of the same compound. The team, led by CFEL scientist Prof. Jochen Küpper from the Hamburg Center for Free-Electron Laser Science CFEL and Prof. Stefan Willitsch from the University of Basel, presents its work in the US journal “Science”.
Many chemical compounds have numerous so-called conformers – different forms of a molecule in which some building blocks are spatially rearranged (rotated). “Even tiny changes in structure can greatly affect the properties of a compound,” says Küpper. “We want to know how exactly the molecular structure influences chemical reactions. Different conformers can even lead to different reaction products.”
To investigate these differences in more detail, the researchers have built a sorting machine for molecules, which can be used to selectively pick a conformer and feed it into a chemical reaction. The device exploits the fact that generally a change in structure also modifies the so-called dipole moment of a molecule. The dipole moment describes how a molecule interacts with an electric field. The sorting machine generates a non-uniform electric field between an electrically charged rod and a trough. When the researchers send several conformers at once through the narrow slit between the rod and the trough, the field deflects the various conformers to different degrees. The device thus provides sorted beams of different conformers, which can be spatially separated and directed individually into a reaction chamber.
As a test, the teams of Küpper and Willitsch sent two conformers of a well-known compound from the group of aminophenols through the device for sorting. Aminophenols are organic compounds that are used, among other things, for the production of medicines and dyes. The selected conformers differed only in the direction in which a single hydrogen atom is attached to an oxygen atom. “As a result, one conformer has a three times larger dipole moment than the other,” says Küpper.
The scientists directed the various sorted beams alternately into a so-called ion trap developed in Basel, in which the two conformers would react with electrically charged calcium atoms (calcium ions). “In the ion trap, the calcium ions are cooled to almost absolute zero at a temperature of minus 273 degrees Celsius. Doing so, they arrange themselves in space and form an ideal target for reactions with the spatially separated conformers,” says Willitsch.
“It turned out that one of the conformers has a reaction rate that is twice as high as that of the other,” says team member Yuan-Pin Chang from CFEL. A tiny change in structure thus leads to big differences in chemical reactivity. “In this way, the method allows insights into fundamental reaction mechanisms that can lead to more effective syntheses of new molecules, such as active pharmaceutical ingredients,” says Willitsch.
As a next step, the researchers want to improve the ion trap so that various ions and molecules could be used as reactants for sorted conformers. In addition, the trap will be combined with a mass spectrometer to determine the reaction products more accurately.